Atomic mass unit (a.m.u.)
The atomic mass unit (u) is the unit of mass for atoms and subatomic particles such as the proton, neutron and electron.
1 a.m.u or 1 u is![]() of the mass of the
of the mass of the
carbon-12 atom.
1 u = 1.66 x 10-27 kg
Example 1
The mass of an atom Cobalt-60 is 59.933820 u.
What is the mass of the atom in kilogram?
Solution
Nuclear Fission
Nuclear fission is the splitting of a heavy nucleus into two lighter nuclei, which subsequently emit either two or three neutrons and release of large amounts of energy.
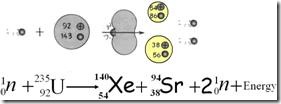
The example of a nuclear fission is shown as follow:
When a uranium-235 is bombarded by a neutron , it is split into two fission fragments (Kripton-131 and Barium-142 ) and three free neutrons.
Another example of nuclear fission is :
Chain reaction
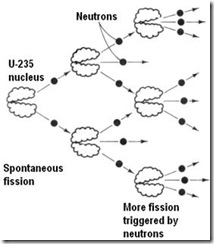
A reaction that is self sustaining as a result of the products of one step initiating a subsequent step.
In nuclear chain reactions the succession depends on the production and capture of neutrons.
Thus, one nucleus of isotope uranium-235 can disintegrate with production of two or three neutrons, which cause similar fission of adjacent nuclei. These in turn produce more neutrons which go off and split other uranium atom – and so on.
A controlled chain reaction is used in nuclear power stations while an uncontrolled chain reaction is used in nuclear bombs.
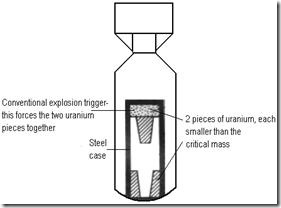
Critical mass
The minimum mass of fission material that will sustain a nuclear chain reaction.
For example , when a nucleus of uranium-235 disintegrates two or three neutrons are released in the process, each of which is capable of causing another nucleus to disintegrate , so creating a chain reaction. However, in a mass of U-235 less than the critical mass, too many neutrons escape from the surface of the material without hitting , preventing a chain reaction from happening.
In the atom bomb, therefore, two or more sub-critical masses have to brought together to make a mass in excess of the critical mass before the bomb will explode.
Nuclear Fusion
Nuclear fusion is the combining of two lighter nuclei to form a heavier nucleus with the release of large amount of energy.
The example of a nuclear fusion is shown as follow:
Nuclear fusion is believed to be process by which energy is released by the Sun. When two
hydrogen-2 nuclei moving at high speed collide, they can join together to produce a heavier nucleus. A large amount of energy is released.
The temperature of a gas must be high giving a high average kinetic energy. Due to the requirement of high temperature, nuclear fusion is also known as a thermonuclear reaction.
Hydrogen bombs are made following the principle of nuclear fusion.
Another example of nuclear fusion is :
Nuclear Energy
According to Albert Einstein, In a nuclear reaction (nuclear fission and fusion) neither mass nor energy are conserved separately but they can exchanged one for the other and only the “mass-energy” is conserved. A loss of mass means that the mass has changed to energy.
The relationship between the mass and the energy is given by the equation
E = mc2
Where
E = energy released
m= loss of mass or mass defect
c = speed of light = 3 x 108 ms-1
Example 2
Polonium-210 undergoes alpha decay to become plumbum-206 . The equation for the decay is:
Additional information:
Mass Po = 209.982 u
Mass Pb = 205.969 u
Mass He = 4.004 u
1 u = 1.66 x 10-27 kg
c = 3 x 108 ms-1
Using the equation and the information above , calculate
(a) The mass defect
(b) The energy released
(c) The power generated in 2 ms
Solution
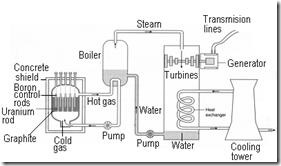
Generation of electricity from nuclear energy – Nuclear Power Station
The energy released from nuclear fission can be used to generate electricity. A nuclear power station consists two main components:
(a) Nuclear reactor
(b) Generator
|
Component |
Function |
|
Graphite core |
Acts as moderator to slow down the fast neutrons produced by the fission. In some nuclear power plant, the moderator is water. |
|
Uranium rod (fuel) |
To produce nuclear power when the fission reactions occur in the uranium rod |
|
Boron control rod |
To control the rate of fission reaction. The control rods are lowered into the reactor core to absorb some of the neutrons and thus reduce the rate of the fission reaction. Sometimes the rod is made of cadmium |
|
Coolant |
To take away heat from the nuclear reactor. ‘Heavy’ water and carbon dioxide are used as coolant because they have high specific heat capacity. |
|
Concrete shield |
To prevent leakage of radiation from the reactor core |
The main components of generator :
|
Component |
Function |
|
Steam generator |
To change water into steam when the water in the generator is heated. The steam then drives the turbines |
|
Turbine |
To turn the coils in the dynamo in the electrical generator to produce electricity |
The pros and cons of using nuclear fission to generate electricity
Nuclear power is controversial. Here are some arguments for and against using nuclear power station to generate electricity.
- Nuclear power provides cheaper electricity than any other method because the nuclear power stations need less fuel than stations which use fossil fuels.The price of nuclear fuel is more stable than fossil fuels.Vast reserves of nuclear fuel in the world.
- Safety procedures in the administration of nuclear reactors are very advanced and safe. Workers in nuclear power stations are at less risk than those in other energy industries. Many people have been killed in accidents in coal mining and oil rigs; very few comparable accidents have occurred in nuclear power stations.
- Nuclear power is clean because produces less waste than fossil fuels.Burning fossil fuels in power stations does more damage to the environment than nuclear power stations. One of the major causes of acid rain is the sulphur dioxide and nitrogen dioxides released from burning coal in power stations. So nuclear power does not add to the greenhouse effect.
- Produces useful radioisotopes as by products that can be used in industry, medicine, agriculture and research.
1. The initial cost to design and build a nuclear power station is very high.
Used fuel rods are very hot and highly radioactive with very long halve-lives. Expensive procedures are required to cool down the rods and store them.
2. There is always a risk of accidents. If something goes wrong with a nuclear power station , it is very much more serious than an accident at a conventional power station. The effects cross national boundaries and can be felt many hundreds of kilometres away.
The hot water discharged from the nuclear power stations can be caused thermal pollution.
People who work in the nuclear power station and those living nearby may be exposed to excessive radiations.
The arguments about nuclear power do not lead to any clear conclusion. It is not the sort of question which can be resolved and answered by simply looking at the facts. For some people one of these points is so important that it outweighs all the others. No one can simply weigh up all the evidence and arrive at the ‘right’ decision. There will always be room for discussion and argument.
Filed under: Uncategorized |










 Bureau international des poids et mesures
Bureau international des poids et mesures
Leave a comment